Mineralocorticoid receptor antagonists (MRAs) in HFrEF or post-MI LV dysfunction (EPHESUS, EMPHASIS, RALES)
EMPHASIS-HF: Zannad F, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11-21.
Bottom-line:
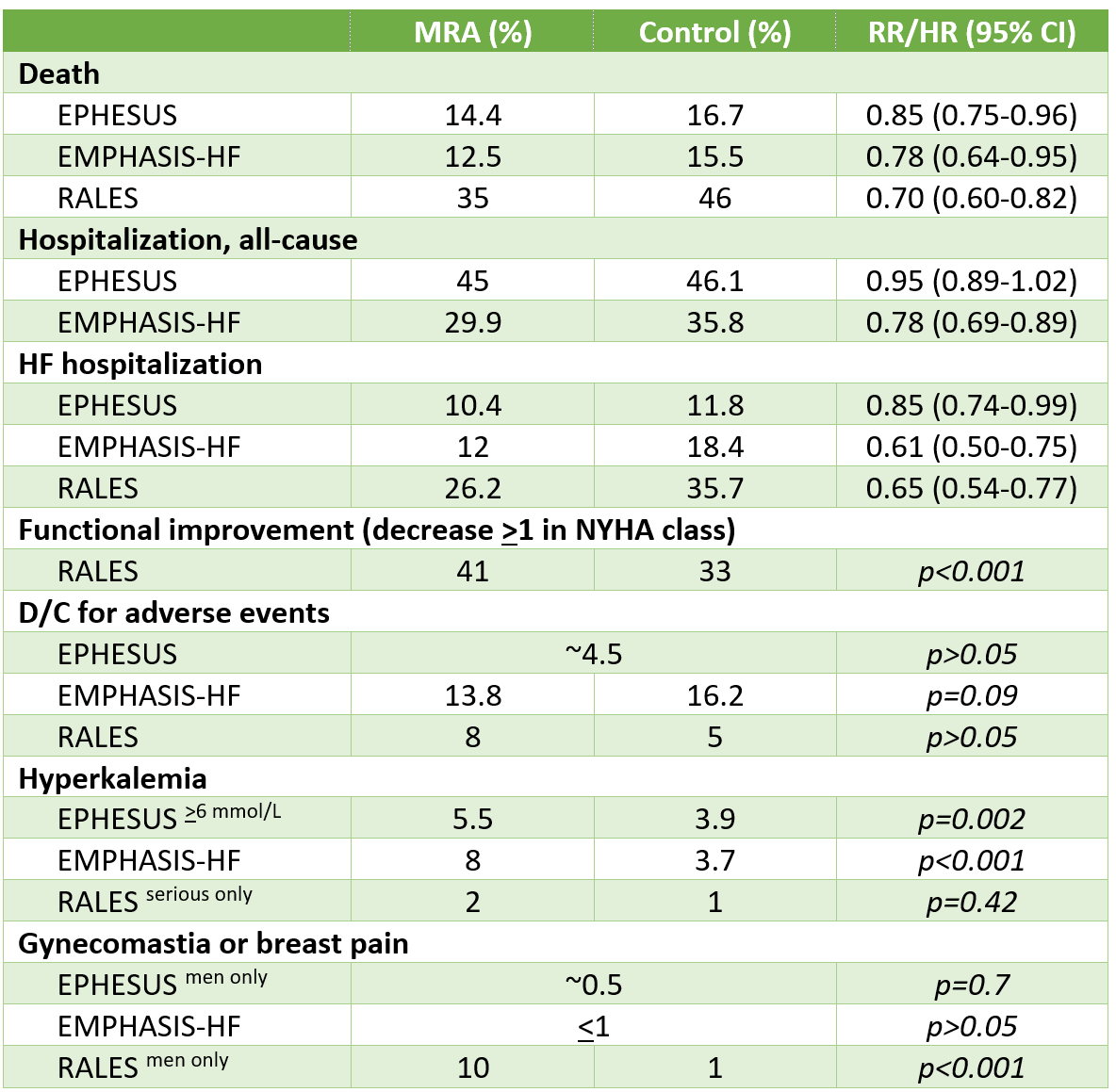
In patients with HFrEF with NYHA functional class 2-4, MRAs reduced the risk of death (NNT 18-60 per year) and hospitalization (NNT 24-30 per year), but increased the risk of hyperkalemia.
Proper monitoring of renal function & serum potassium (e.g. 1 week after start/dose change, monthly x3 months, then q3-6 months) is critical to ensure that harms do not outweigh benefits of this important therapy.
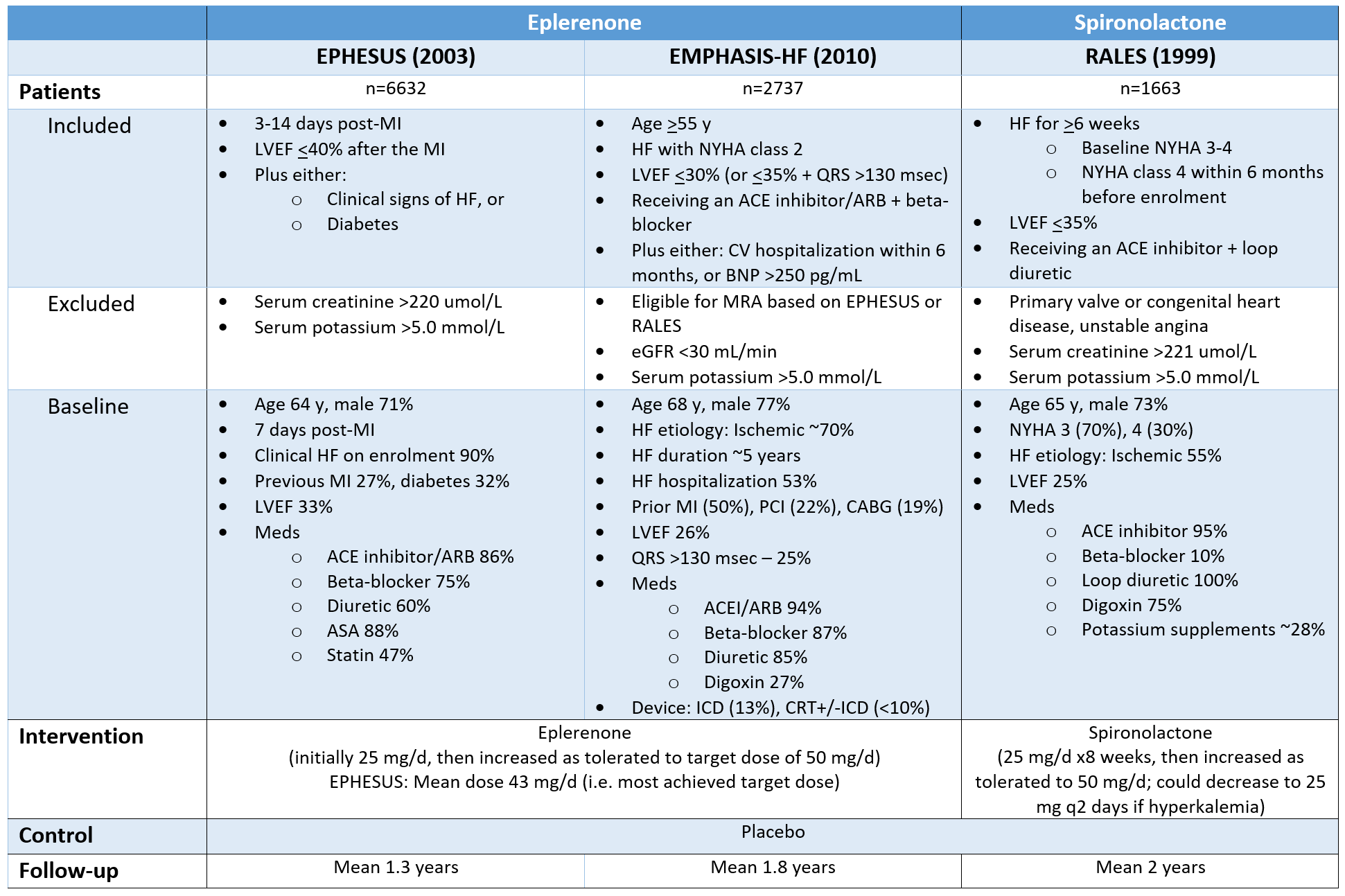
Patients, Interventions, Controls & Duration of Follow-Up
Results
Additional Results/Analyses
Results expressed as a difference in mean survival time if therapy was started at 60 y/o
EMPHASIS-HF: Survival without CV death/HF hospitalization:
Placebo ~6 years, eplerenone 8.9 years (difference 2.9 years)
EPHESUS: Survival without CV death/CV hospitalization:
Placebo 3.6 years, eplerenone 4.8 years (difference 1.2 years)
Generalizability & internal validity
RALES set the indication for mineralocorticoid antagonists in HFrEF NYHA class III-IV; EPHESUS expanded it to HFrEF or LV dysfunction+diabetes post-MI; EMPHASIS-HF further expanded the indication to HFrEF NYHA II with high risk for HF hospitalization.
Assessed together, these 3 trials evaluated MRAs in all stages in a varity of etiologies for HFrEF, as early as 3 days post-MI, with background therapy ranging from the full gamut to only ACEI + diuretic therapy.
Trials employed routine monitoring for renal & potassium abnormalities:
EPHESUS: Serum potassium 48h after treatment start, then at weeks 1, 4, 5, 12 of treatment, then q3 months
RALES: Serum potassium q4 weeks x3 months, then q3 months x1 year, then q6 months
Internal validity: Low risk of bias in all 3 trials